Steriliant is your one stop shop for sterile packaging product development and project management.
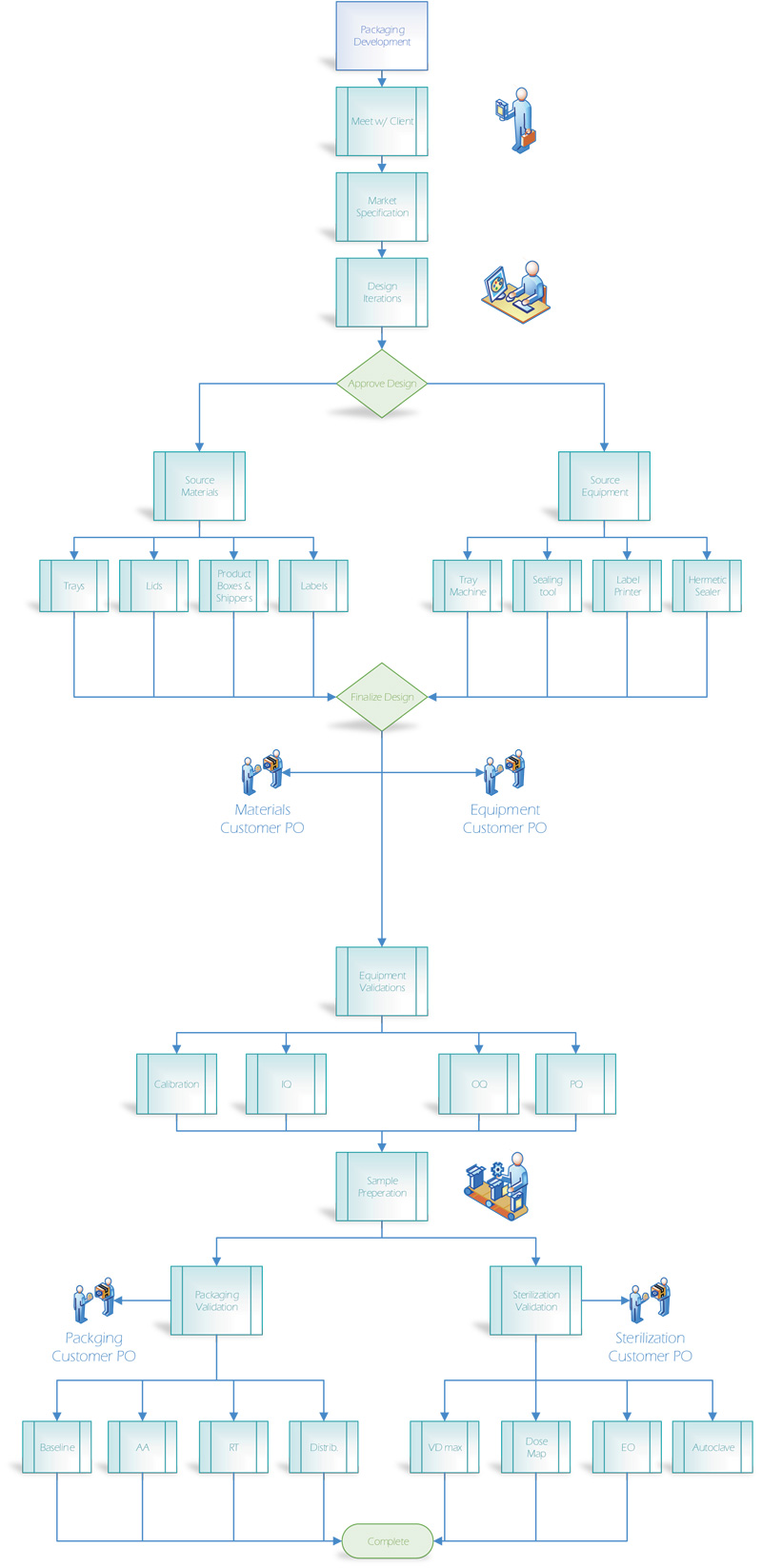
If you manufacture a medical device to be sold sterile and lack the in-house resources to develop the packaging, we can help. We source materials & equipment, and validate and manage all development activities of sterile packaging, including protocol and report drafting and packaging/sterilization validation testing. We also offer product design to include 3D CAD models and drawings, always keeping manufacturability in mind.
Steriliant is a San Diego based company founded by a group of development engineers/project managers with an extensive background in packaging, biologics, orthopaedics, and design engineering.
With years of experience, we’ve grown knowledgeable of all the testing requirements, the vendors that can do the testing and provide the materials and equipment, and the documentation you need to have for your design history file.
We’ll work with you to develop a reasonable timeline to get all the work done.
Milestones along the way will chart your progress, and you’ll never be in the dark as to where you are in the process. Your success is our success, and we take our success very seriously!
